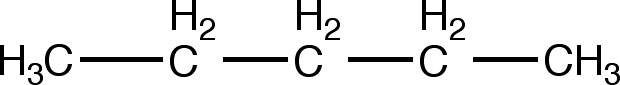

A carbon chain is a line of carbons, where each carbon is bonded to at least one other carbon atom. A carbon chain may contain single, double, or even triple bonds.
In order to name an organic compound you must first count the number of carbons in the longest carbon chain.
note: If the organic structure contains a functional group count the longest carbon chain from the functional group.
When you have determined what the longest carbon chain is, locate the corresponding stem:
| Longest C chain | Stem |
| 1 | meth |
| 2 | eth |
| 3 | prop |
| 4 | but |
| 5 | pent |
| 6 | hex |
| 7 | hept |
| 8 | oct |
| 9 | non |
| 10 | dec |
| 11 | undec |
| 12 | dodec |
For carbon chains greater than 12 use the stem "dec" and the other stem as a prefix
Ex) 16 carbons= hexadec
Ex) 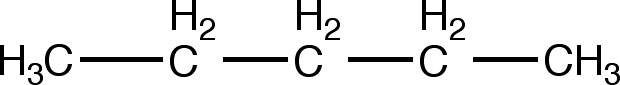
For this example we would use the stem pent
| TYPE OF BOND | ENDING |
| single | -an |
| double | -en |
| triple | -yn |
Let's use the previous example
All of the carbon atoms are connected to each other by single bonds, therefore we would add the ending -an making it a pentane group. Easy as pie!
If our structure contained a double bond, like so:

We would use the -en suffix, making it a pentene group. Since merely stating that the structure is a pentene group does nothing to notate the position of the double bond, we use a numeric system. In our example the double bond starts at the second carbon atom and continues into the third. Therefore, we would name this pentene group 2-pentene. You're doing great! Let's try one more example.
Try this one on your own!
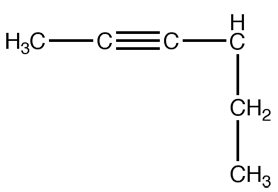
Very good! Did you realize that it is a hexyne group this time? I bet you did. In fact it is not only a hexyne group it is 2-hexyne.Ready for step 3?
This step is easy! An alkyl group is just the same as its stem minus a hydrogen atom. You can recognize an akyl group through its distinctive -yl ending.
So on and so forth!
note: Before attempting to name a compound that contains a functional group make sure you understand what a funtional group is and are able to identify them.
| FUNCTIONAL GROUP | SUFFIX |
| alcohol (-OH) | -ol |
| aldehyde (-COH) | -al |
| ketone (-CO-) | -on |
| carboxylic acid (-COOH) | -ic acid |
| Ester (-COO-) | ester |
| Ether (-O-) | ether |
| Amine | * amino- |
| ** Halides | stem-o |
* amino- is to be used as a prefix not a suffix unlike other functional groups.
** a halogen is not technically a functional group but in order to note them in organic compounds we must treat them as such.