Log In
 Ralph & Bev Shock
Ralph & Bev Shock 
Chapter 4
Atomic Structure
Scientists often use models to visualize structures that can not be easily seen with the unaided eye. These model are usually based on experimental data.
For example in 1913 Niels Bohr was studying the interactions between light and matter and determined that atoms give off specific
wavelengths of light when excited by discrete amounts of energy. His research and previous research and experimentation led to the classic Bohr model
of the atom; A nucleus composed of positively charged protons and neutral neutrons surrounded by specific orbitals or energy levels where electrons
of a certain energy level can be found. The analogy is like a miniature solar system.
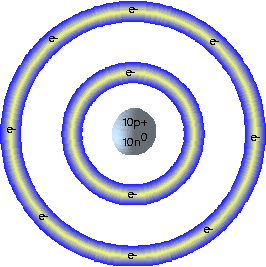 * Neon
* Neon
The story of atomic theory really started much earlier in fact in 440 BC, the Greek philosopher Democritus hypothesized that
all matter is made of indivisible particles called atoms which in the Greek means uncutable. Although atomism continued to
be debated for nearly 1400 years it was not until the scientists of the 19th century, such as Lavoisier, Dalton and Mendeleev
that the chemistry of elements started to take shape and it was realized that certain substance could be divided down to their
“atomic” size and still retain all the chemical and physical properties of that substance.
In the 1800's John Dalton proposed an atomic theory of matter. His theory had four main points. (1) all matter is composed
of atoms which cannot be broken apart; (2) all atoms of the same elemnts are identical in mass and in chemical properties;
(3) atoms of different elements are different in mass and in their chmical properties; (4) atoms combine in whole number
ratios to form compounds.
Dalton stated that in any given chemical compound, the elements always combine in the same proportion by mass.
This statement is the law of definite proportions.
Of course Dalton was unaware of isotopes and subatomic particles and has be modified accordingly.
The first real breakthrough that led to the understanding of the composition of the atom came from the experiments of J.J Thomson (1856-1940).
On April 30, 1897, Joseph John (J.J.) Thomson (1856-1940) [13K GIF] announced that cathode rays were negatively charged particles
which he called 'corpuscles.' He also announced that they had a mass about 1000 times smaller than a hydrogen atom, and he claimed
that these corpuscles were the things from which atoms were built up. Later in 1897, he wrote:
"...we have in the cathode rays matter in a new state, a state in which the subdivision of matter is carried very much
farther than in the ordinary gaseous state: a state in which all matter-that is, matter derived from different sources such
as hydrogen, oxygen, etc.-is of one and the same kind; this matter being the substance from which the chemical elements are
built up." (J.J. Thomson (1897). "Cathode Rays," Philosophical Magazine 44, 295.)
He had came to the conclusion that the particles in the cathode ray (which we now call electrons) were a fundamental part of all matter. Later he would recall:
“At first there were very few who believed in the existence of these bodies smaller than atoms. I was even told long afterwards by a distinguished physicist
who had been present at my [1897] lecture at the Royal Institution that he thought I had been `pulling their legs.' " (J.J. Thomson (1936). Recollections
and Reflections. G. Bell and Sons: London. p. 341.)
Thomson who studied electrical discharges in partially evacuated tubes called cathode-ray tubes found that when high voltage was applied to the tube rays
emanated from the cathode (negative electrode) to the anode (positive electrode). These rays could be deflected by either an electrical or magnetic field.
By adjusting the electric field to the cathode rays he determined the charge-to-mass ratio of the particle we now call the electron.
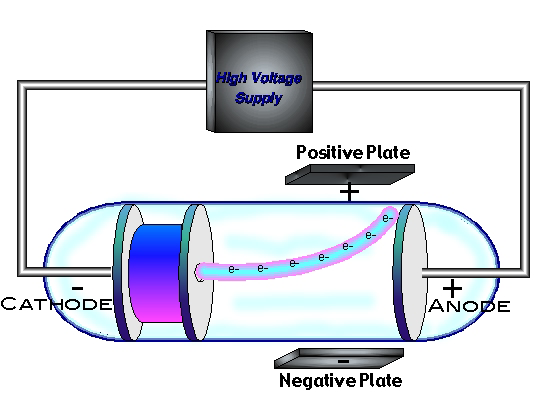
Since all non nuclear chemistry involves transfer and or sharing of electrons, Thomson’s discovery paved the way for understanding chemistry
at the atomic level and inspired continued euclidiation of atomic structure by Ernst Rutherford , Goldstein, Chadwick , and Marie Curie.
In 1886, Golstein observed rays traveling in the opposite direction of cathode rays. Golstein named these canal rays. This would later be found
to be made up protons.
In the early 1900's Marie Curie 's and her husband began to study the radioactivity of uranium. Her experiments led her to believe that not
all the radioactivity could be explained by uranium.
The Curie's began a search for the source of the radioactivity and discovered two highly radioactive elements, "radium" and
"polonium." The radiation was cause by what Rutherford later determined to be alpha particles. The Curie's won the 1903
Nobel prize for physics for their discovery. They shared the award with another French physicist, Antoine Henri Bacquerel, who had
discovered natural radioactivity. In 1906 Pierre, overworked and weakened by his prolonged exposure to radiation, died when he
was run over by a car.
Madame Curie continued her work on radioactive elements and won the 1911 Nobel prize for chemistry for isolating radium and studying
its chemical properties. In 1914 she helped found the Radium Institute in Paris, and was the Institute's first director.
When the first world war broke out, Madame Curie thought X-rays would help to locate bullets and facilitate surgery.
It was also important not to move the wounded, so she invented X-ray vans and trained 150 female attendants.
The radioactivity was the result of beta particles, a particle with a mass of 4 amus the same mass as a helium nucleus.
Ernest Rutherford was a friend of the Curies, and people like Einstein, Lorentz, and Planck. He was said to be their "best buddies"
and the life of the party. Inspired by their research Rutherford began working on his own theory of the atomic model.
Rutherford's ground breaking experiments with studying alpha particles. With his colleges Hans Geiger and Ernest Marsden they aimed
a stream of alpha particles at a thin gold foil. The foil was so thin that it had to be supported on a glass plate.
(The plate without any foil was studied and no deflections were found. It was transparent to the alpha particles.)
There were three major findings:
1) The vast majority of the alpha particles went through the gold foil as if it were not even there. Those alpha particles,
of course, continued on a straight-line path until they hit a phosphorescent detector screen.
2) Some of the alpha particles were deflected only slightly, usually 2° or less.
3) A very small fraction of alpha particles were deflected by an angle of 90° or more. (Rutherford cites 1 in 20,000 for gold in his 1911 paper.)
The following diagram illustrates Rutherfords experiments and findings. R is the source of alpha particles and F is the foil that scatters the alpha particles.
M is the microscope used to look at the detector screen which was attached to the front of the microscope.
In order to see the flashes on the screen a the experimenter would have the let their eyes adjust to the dark for more than an hour.
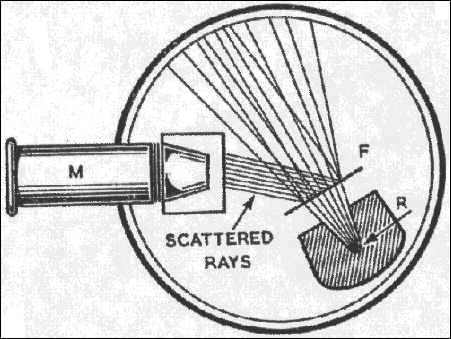
Rutherfords experiments disproved the "plum pudding" model as put forth by Lord Kelvin William Thomson where
the electrons are like raisins dispersed in a "pudding" of positive charge. Rutherfords experiments showed
that atoms are mostly empty space with a nucleus of positive charge and high density.
The Next major breakthrough in atomic structure was based on the experiments of the Danish physicist Neils Bohr.
Bohr knew from classical Newtonian physics that a particle in motion tends to move in straight line and can be
made to travel in a circle only by the application of a force towards the center of the circle; like gravity
pulling on a satellite orbiting the earth. If such and object does not has force maintaining its velocity it
will fall towards the center do the force causing it to move in a circle.
Bohr also knew from classical physics that a a charged particle under acceleration should produce electromagnetic
radiation. An electron revolving around a nucleus is constantly changing direction therefore it is accelerating
and should emit light energy. This loss of energy should cause an electron to spin closer and closer to the
nucleus until it "crashes" into the nucleus. This is not what we observe in nature therefore the
physics of atoms cannot be explained by newtonian physics.
Bohr observed in his experiments with the hydrogen atom that only a descrete amount of energy will excite and electron
and cause it to move to a higher energy level. When the electron "falls back" to a lower energy level it
gives off a descrete amount of energy in the form of light equal to the amount that excited the electron in the first
place. This light emitted by the electrons falling back to lower energy stated produced a line spectrum instead of a
continual spectrum as predicted by Newtonian physics. His data showed that only certain electron energies
"orbits" were allowed and that electrons could not exist in-between these orbits.
We now know that a line spectra is like a fingerprint for the elements, each element having its own unique spectra
that corresponds to a map of the energy levels that electrons can exist in for that element . The electron can move
from its ground state (low energy state) to a higher energy state when it absorbs a photon, energy of a specific
frequency. When an "excited" electron drops from this orbit to a smaller one, a descrete amount of energy,
a quanta, is radiated as a photon of a specific wavelength of light.
Isotopes:
As we look at the periodic table we notice that each time the atomic number changes the element changes.
The atomic number of an element represents the number of protons in an atom; therefore we must conclude
it is the number of protons in an atom that determines the identity of an element.
As the elements appear on the periodic table they are electrically neutral, the number of electrons must
equal the number or protons. As we will see later it is the affinity of an element to gain, loose, or share
that determines their chemical properties but the number of electrons around an atoms nucleus does not determine
what the element is.
Isotopes are atoms of an element that are exactly alike chemically by differ by their masses. Isotopes have the
same number of protons but different numbers of neutrons. The atomic mass of an element is the sum of its protons
and neutrons. Since protons determine the element, the masses of isotopes differ and it is the number of neutrons
that determine a particular isotope of an element.
The numbers of neutrons can be calculated by subtracting the atomic number (number of protons) from the mass number.
atomic mass - atomic number = number of neutrons
carbon-12 has a mass of 12 atomic mass units
carbon has an atomic number of 6 (protons)
12-6=6 neutrons
Carbon has other isotopes such as carbon -14 a radioactive form of carbon with a mass of 14 therefore, it has: 14(mass) -6 (protons)= 8(neutrons)
Because the number of neutrons does not change the chemical properties of an element, carbon-14 is used in many
biological experiments with living organisms. The fact that it is radioactive allows scientists to track or
"trace" the movement of carbon containing elements in biological pathways. This helps them determine
how and where biochemicals are being synthesized and used.
In the symbol of an isotope the mass is indicated as a preceding superscript and the atomic number as a preceding subscript.
146C
Name: Carbon-14
Atomic Mass:
The actual mass of atoms and atomic particles are very small. Hydrogen the lightest element consisting of only one proton and one electron is 1.67x10-24 grams. It would be very inconvenient to represent the elements masses on the periodic table in grams. Because of this impracticability scientist have defined a mass scale for atomic and subatomic particles based on a new reference scale. The atomic mass scale is arbitrarily based on 1/12 the mass of a carbon-12 atom. The unit of this scale is the atomic mass unit (amu). The atomic weights on the periodic table are based on this system. As we will see later this relationship is important for predicting the the amount of one element or compound will react with another element or compound.
Average Atomic Mass:
As can be seen on the periodic table the atomic weights are not whole numbers. This is because
in nature a sample of an element contains more that one isotope of that element. The atomic weights
on the periodic table are weighted averages of the isotopes for each element as found in nature.
In other words it is the average weight based on the abundance of each isotope.
Example: The relative abundances of isotopes for chlorines are:
75.771% chlorine-35
24.229% chlorine-37
Each of the isotopic masses is multiplied by its factional abundance. The products are then added to give the weighted average.
35.000 x 0.75771 = 26.51985 amu's
37.000 x 0.24229 = 8.96473 amu's
______________________
sum=35.48458 amus
Practice Problem: Calculate the average atomic weight of magesium:
Relative abundance of isotopes:
Magnesium-24 (78.70%)
Magnesium-25 (10.13%)
Magnesium-26 (11.17%)
Answer: 24.324 amu's
Example Test: Chapter 4
I. MULTIPLE CHOICE: On the answer sheet mark the BEST response to each of the following.
_______1. Which of the following is not part of Dalton's atomic theory? (a) all elements are composed of atoms, (b)
atoms are always in motion, (c) Atoms of the same element are alike in mass and size, (d) atoms that combine do so in
simple whole number ratios.
_______2. The nucleus of an atoms is (a) positively charged with a high density, (b) positively charged with a
low density, (c) negatively charged with a high density, (d) negatively charged with a low density.
_______3. The number of neutrons in the the nucleus of an atom can be calculated by (a) adding the number of
electrons and the number of protons, (b) subtracting the number of electrons from the number of neutrons, (c)
subtracting the number of protons from the mass number, (d) adding the mass number and the number of electrons.
_______4. All atoms of the same element have the same (a) number of neutrons, (b) number of protons, (c) mass numbers,
(d) atomic mass.
_______5. The number 84 in the name krypton-84 represents (a) the atomic number (b) the mass number (c) the sum
of protons and electrons, (d) none of these.
_______6. Which of these statements is false? (a) atoms of the same element may have different masses, (b)
atoms of isotopes of an element have different numbers of protons, (c) the nucleus has a positive charge,
(d) atoms are mostly empty space.
_______7. The mass of atoms are most conveniently measured in (a) amus, (b) grams, (c) angstroms, (d) nanograms.
_______8. If E is the symbol for an element which two of the following symbols represents isotopes of the same element? [(1) 1020E (2) 1120E (3) 921E (4) 1021E ]
(a) 1 and 2, (b) 3 and 4, (c) 1 and 4, (d) 2 and 3.
_______9. Average relative atomic masses are measured in: (a). amus (b) grams (c) angstroms (d) nanograms
Complete these tables:
| Atomic number | Mass Number | Number of Protons | Number of Nuetrons | Number of Electrons | |
| #10 | 8 | ______ | ______ | 8 | ______ |
| #11 | ______ | 14 | ______ | 7 | ______ |
| #12 | ______ | ______ | ______ | 21 | 20 |
| #13 | 11 | 23 | ______ | ______ | ______ |
| #14 | ______ | 56 | 26 | ______ | ______ |
| Protons | Neutrons | Electrons | Name of nuclide | ||
| #15 | 613C | ______ | ______ | ______ | ____________ |
| #16 | 715N | ______ | ______ | ______ | ____________ |
| #17 | 1020Ne | ______ | ______ | ______ | ____________ |
| #18 | 511B | ______ | ______ | ______ | ____________ |
| #19 | 49Be | ______ | ______ | ______ | ____________ |
20. Two atoms of the same element can have different numbers of _______________.
21. The nucleus is composed of neutrons and _______________.
22. The number 84 in 84Kr is called the _______________.
23. Sir James Chadwick is given credit for the discovery of the _______________.
24. The _______________ of an element is the weighted average of the isotopes of that element.
25. The _______________ gives the atom its volume.
26. Dalton stated that all atoms of the same element are _______________.
27. When electrons were first discovered they were called _______________ rays.
28. _______________ reactors are nuclear reactors that have the potential to explode.
29. Proton and neutron belong to a family of atomic particles called hadrons. Hadrons consist of smaller particles called ____________.
Matching
COLUMN A
___ 30. proton
___ 31. atom
___ 32. mass number
___ 33. atomic mass unit
___ 34. electron
___ 35. isotopes
___ 36. atomic number
___ 37. atomic mass
___ 38. nucleus
___ 39. neutron
COLUMN B
a. the smallest particle of an element that retains the properties of that element
b. the weighted average of the masses of the isotopes of an element
c. 1/12th of the mass of a carbon atom with six protons and six neutrons
d. the number of protons in the nucleus of an atom
e. a negatively charged subatomic particle
f. atoms with the same number of protons but different number of neutrons
g. the total number of protons and neutrons in the nucleus of an atom
h. subatomic particle with no charge
i. subatomic particle with no charge the central part of an atom, containing protons and neutrons
j. a positively charged subatomic particle